Abstract
Background: Recent large-scale next generation sequencing studies have revealed a highly heterogeneous genetic landscape in CLL. However, the majority of the genetic lesions are relatively infrequent and their functional role and clinical relevance are still unknown. Moreover, in vivo models to study how these lesions cooperate between themselves and affect the response to novel therapeutic agents are still lacking. To further understand the relative contribution of individual genetic lesions to CLL development, progression and response to treatment, we performed multiplex CRISPR/Cas9-editing of normal and leukemic murine B cells by targeting a large set of CLL driver genes.
Materials and Methods: Multiplexed CRISPR/Cas9 editing was done on normal B1 and B2 B cells isolated from the peritoneal cavity of young (<4 months) del(13q14)MDR-/- mice or on leukemic B1 B cells isolated from the spleen of old (>12 months) Eμ-TCL1 mice using a recently established procedure (Chakraborty et al, Blood 2021). The effects of the introduced genetic lesions on leukemia cell growth and treatment resistance were investigated by analyzing the mutant allele frequency (MAF) of the injected cells and cells recovered from the spleens of untreated or venetoclax-treated mice.
Results: To investigate whether multiplex CRISPR/Cas9 editing can be used to transform normal murine B cells derived from del(13q14)MDR-/- mice, we simultaneously targeted 12 putative CLL driver genes representative of cellular pathways that are commonly affected in CLL, including DNA damage response (TP53, ATM and POT1), chromatin modifiers (CHD2, SETD2), cell cycle (RB1), inflammation (SAMHD1) and the NF-kB (BIRC3, NFKBIE, TRAIL-R), NOTCH (FBXW7) and MYC pathways (MGA) (n=4 experiments). The transfected B1 and B2 B cells were transplanted in the peritoneal cavity of B cell-deficient NSG mice and analyzed at different timepoints within a range of 1 to 5 months. Both cellular subsets were detected in the peritoneal cavity of NSG mice at the initial timepoint, but B1 B cells preferentially expanded at the later timepoints, giving rise to CD19+/CD5+ leukemias 3-5 months after transplantation. An increase in MAF was observed for all targeted genes, suggesting that all of the introduced genetic lesions provide a growth advantage to the leukemic cells.
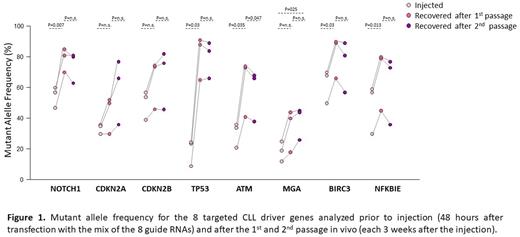
To investigate the impact of selected genetic lesions on CLL progression and Richter transformation (RT), we simultaneously introduced activating mutations in NOTCH1 (targeting of the PEST domain) or inactivating mutations in MGA, ATM, TP53, CDKN2A, CDKN2B, BIRC3 and NFKBIE in CLL cells derived from Eμ-TCL1 transgenic mice (n=3 independent experiments). A rise in MAF was observed for all 8 genes following in vivo propagation of the edited cells, suggesting that all of these mutations are positively selected during leukemia progression. However, selection of NOTCH1, TP53, ATM, BIRC3 and NFKBIE mutations occurred more rapidly, reaching the maximum MAF already after the 1st passage in vivo, whereas the MAF of genetic lesions specifically associated with Richter transformation, i.e. CDKN2A, CDKN2B and MGA, rouse more slowly and increased during serial passages (Figure 1). Interestingly, a substantial increase in MAF was observed for CDKN2A and CDKN2B when cells from the 1st passage were placed in culture (from 44% to 93%, p=0.017, and from 64% to 94%, p=0.043, after 6 weeks in culture for CDKN2A and CDKN2B, respectively), consistent with our recent finding that biallelic disruption of these genes allows for spontaneous proliferation of Eμ-TCL1 leukemia cells and may represent the transforming factor in a subset of cases with Richter Transformation. In contrast, the MAF of the other five driver genes showed no or only minor changes during culture. The MAF of CDKN2A and CDKN2B also increased to a greater extent in mice treated with venetoclax compared to untreated mice, indicating that cells with such genetic lesions are more resistant to venetoclax treatment.
Conclusions: This study shows that multiplex CRISPR/Cas9 editing can be used to rapidly generate murine leukemias with the genetic make-up of human CLL or Richter Syndrome. These models can represent an important preclinical tool to study how individual genetic lesions cooperate during CLL development and progression and how they affect the response to treatment with novel therapeutic agents.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal